
For example, according to the Aufbau principle, the configuration of Fe(Z = 26) is 1s 2,2s 22p 6,3s 2,3p 6,4s 2,3d 6, it has been confirmed by the spectral and magnetic studies of Fe 2+ that Fe 3+ has configuration 1s 2,2s 22p 6,3s 2,3p 6,4s 2,3d 6, and not 1s 2,2s 22p 6,3s 2,3p 6,6s 2,3d 4, which shows that ionization cause the loss of electrons in preference to electrons even though the loss to be added in building up the configuration of Fe-atom. In other words, It fails to describe which electrons are removed when a cation is formed from an atom.
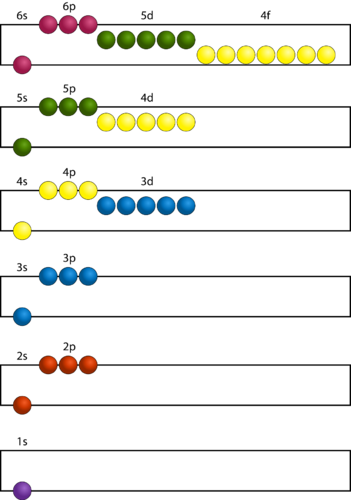
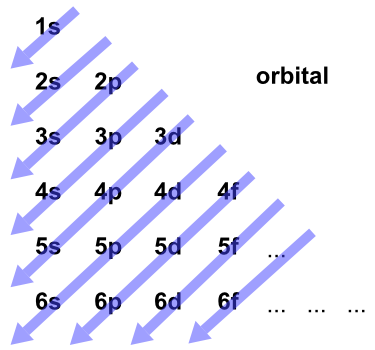
Related Topic | Atomic Structure and Periodic Table Thus, the order of energies of the orbitals will obtain by increasing (n + l) values.ġs 90 is 1s < 2s < 2p < 3p < 4s < 3d < 4p < 5s < 4d < 5d < 6s < 4f < 5d < 6p < 7s < 5f < 6d.

The orbitals for which the value of (n + l)is lowest, are fined first by the electrons. Using n and l values he was able to derive the same sequence of energy levels by applying the following rules :ġ. Madelung suggested that the above precise order which is followed in the filling up of the orbital is based on the principle quantum number ‘ n‘ and the azimuthal quantum number ‘ l‘. For the sake of simplicity, Moeller In 1952 suggested a “mnemonic diagram’ (figure 1.16) that gives the same order of energy levels. This complete order is not only correct for a single element, but it is also remarkably accurate for all elements.


 0 kommentar(er)
0 kommentar(er)
